Alcohol produce aldehyde technology is the primary alcohol under the action of catalyst, hydroxyl and adjacent carbon to remove a mole of hydrogen then converted into carbonyl,form the aldehyde with the corresponding number of carbon .
Aldehyde is an important organic raw material and intermediate, quite useful, mainly used as an important intermediate of fine chemical , perfume and medicine. For example: formaldehyde can be used as raw material of phenolic resin, urea-formaldehyde resin, vinylon, ullotropin, pentaerythritol, dye, pesticides and disinfectants ; propanaldehyde is used to make synthetic resin, rubber accelerator and anti-aging agent, etc. It can also be used as antifreeze, lubricant ,dehydrating agent, etc; isopentyl aldehyde is used as synthetic raw material for rubber promoter, organic synthesis,artificial flavor and vitamin E; benzaldehyde is used as raw material for the production of hyacinthin , lauraldehyde lauric acid and benzyl benzoate ; octyl, nonaldehyde, and malonaldehyde can be used as the intermediate of flavor and organic synthesis, etc.
The main methods for the synthesis of aldehydes are: olefin and CO through hydroformylation reaction and alcohol through catalytic oxidation or dehydrogenation to produce aldehydes.
1, Adaptable raw material
primary alcohol
2,Physicochemical properties of catalysts
Item | Index |
Apperance | Black flake solid |
Size,mm | 5*5 |
Bulk density, g/cm3 | 1.4-1.7 |
Intensity, N/cm | >180 |
3,Process conditions
Item | Index |
Reaction temperature ℃ | 300-350 |
Reaction pressure,MPa(g) | <0.1 |
Gas-liquid ratio | 300:1 |
Mass S V,h-1 | 0.5 |
4,Technical flow diagram
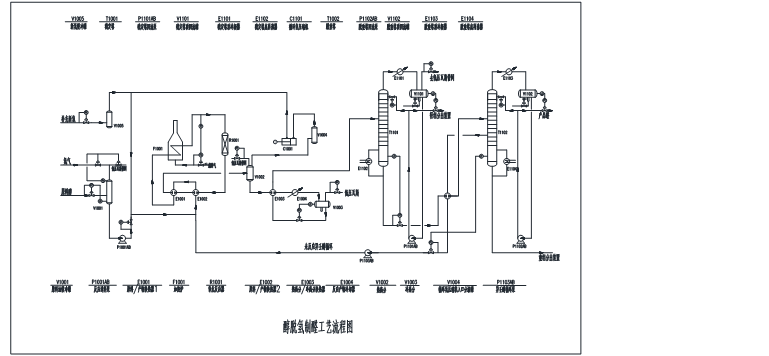
5 ,Process characteristics
Using hydrogen dehydrogenation process, the catalyst life is long, the product aldehyde yield is high, and the by-products are few.The application range of raw materials is wide.